Batch selection
Are there patient-specific differences in ITI outcome with different batches of the same FVIII concentrate?
Potential variation between different batches of a FVIII concentrate could affect how different patients respond to treatment. MOTIVATE aims to understand whether testing different batches of FVIII concentrate against a patient’s plasma in vitro prior to ITI treatment could identify the optimal batch for that patient, to increase their likelihood of ITI success.
Batch selection: Modified Oxford method
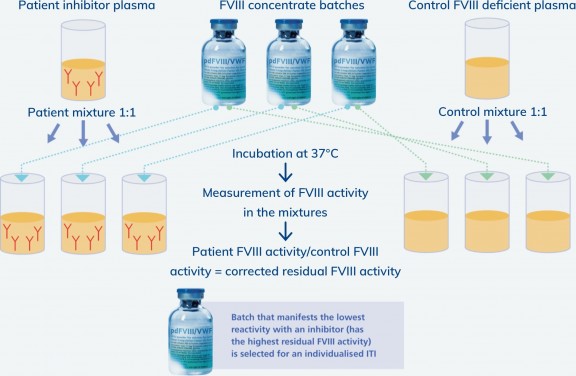
Product-specific batch selection sub-study
Sub-study lead investigator

Carmen Escuriola-Ettingshausen
Director, HZRM Hämophilie-Zentrum Rhein Main,
Mörfelden-Walldorf, Germany
MOTIVATE will assess the effect of batch selection on ITI outcomes in patients receiving ITI with plasma-derived FVIII
What is needed for this analysis?
A blood sample of 3–6 mL at baseline and throughout the trial as needed