Why MOTIVATE?
Development of inhibitors remains a problem in haemophilia A
Neutralising antibodies (inhibitors) against exogenous coagulation factor VIII (FVIII) cause FVIII replacement therapy to be ineffective1.
Bypassing agents can be used to prevent and control bleeding, but their efficacy is less predictable than that of FVIII replacement therapy2.
Haemophilia A patients who develop inhibitors may experience3,4
- Increased risk of bleeding
- Decreased quality of life
- High healthcare costs
- Increased likelihood of progressive arthropathy in joints
- Increased risk of mortality
Immune tolerance induction (ITI) is the only clinically proven strategy to eradicate inhibitors5
Successful inhibitor eradication with ITI allows return to FVIII therapy, enabling:


ITI is recommended for patients with inhibitors by international guidelines
ITI should be started as soon as possible after a
high-titre inhibitor is identified
https://www.hemophilia.org/Researchers-Healthcare-Providers/Medical-and-Scientific-Advisory-Council-MASAC/MASAC-Recommendations/MASAC-Recommendations-on-Standardized-Testing-and-Surveillance-for-Inhibitors-in-Patients-with-Hemophilia-A-and-B
Tolerisation should be tried in all haemophilia A patients with inhibitors of recent onset
Berntorp E et al. <i>Haemophilia</i> 2006; 12:1-7
Non-factor treatment options for patients with inhibitors
Emicizumab is a novel non-factor VIII product that provides bleed protection for patients with or without inhibitors5.
Emicizumab and FVIII are fundamentally different proteins. The only common feature is that they both bring FIXa and FX into close proximity. There are important differences between FVIII and emicizumab in terms of binding affinity, regulation and activity8.
Emicizumab has the benefit of subcutaneous administration5.
However, emicizumab does not eradicate inhibitors and patients on emicizumab still experience bleeds5,9. There is limited experience of major surgery in patients treated with emicizumab and the impact of emicizumab on long-term joint health is unknown10.
ITI therefore remains an important treatment approach.
ITI remains the recommended treatment approach for people with inhibitors
Despite the considerable success of emicizumab in the management of inhibitor patients, the FIT group still sees the importance of eradicating inhibitors. ITI is the only approach that currently offers this potential
Carcao M et al. <i>Haemophilia</i> 2019; 25:676–84. The Future of Immunotolerance Treatment (FIT) Group
Despite the development of nonfactor replacement agents, ITI therapy is likely to maintain a key role in the management of inhibitor patients
Oldenburg J et al. <i>Expert Rev Hematol</i> 2018; 11:857–62
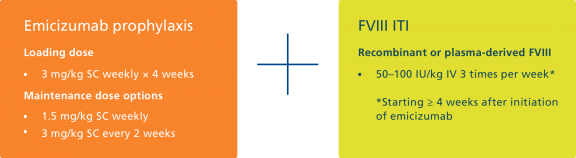
Could we reduce the burden of ITI and achieve inhibitor eradication by combining low-dose ITI and emicizumab prophylaxis?
ITI in combination with emicizumab prophylaxis has been investigated in a small cohort of paediatric patients with haemophilia A and inhibitors, by Batsuli and colleagues in Atlanta11.

Inhibitor titres in seven children on FVIII ITI and emicizumab

This study provided the first evidence that a combination of FVIII ITI and emicizumab prophylaxis is feasible for patients with inhibitors
MOTIVATE is collecting data from real-world clinical experience in patients with haemophilia A and inhibitors
The data collected will allow us to evaluate the impact of different treatment strategies, including ITI in combination with emicizumab prophylaxis.
These data are expected to help inform decisions on evolving the standard of care for people with haemophilia A and inhibitors.
- Rocino A et al. <i>J Clin Med</i> 2017; 6:e46
- Santagostino E et al. <i>Acta Haematol</i> 2019; 141:151-5
- Walsh CE et al. <i>Thromb Haemost</i> 2016; 116:S10-7
- Walsh CE et al. <i>Am J Hematol</i> 2015; 90:400-5
- Carcao M et al. <i>Haemophilia</i> 2019; 25: 676-84
- Young G. <i>Blood Adv </i>2018; 2: 2780-2
- Thornburg CD and Ducore J. <i>Haemophilia</i> 2019; 25:e48-50
- Lenting PJ et al. <i>Blood</i> 2017; 130:2463-8
- Oldenburg J et al. <i>N Engl J Med</i> 2017; 377:809-18
- Knight T and Callaghan MU. <i>Ther Adv Hematol</i> 2018; 9:319-34
- Batsuli G et al. <i>Haemophilia</i> 2019; 25:789-96
References:
